- act as a strong electrolyte and break apart completely into separated cations and anions,
- act as a weak electrolyte and stay principally as intact molecules, but dissociate somewhat into ions,
- act as a nonelectrolyte and stay completely as intact molecules, or
- act as a reactant in a reaction with the water.
At some point you will need to be able to look at just about any chemical formula, classify it into one of the above four classifications. The question will be, "What does this compound do when it is added to water?" In answering that question, you determine the principal species in a solution for that compound. Being able to determine the principal species in solution is one of the major goals of first year chemistry at the college level. Only with this understanding can one start to see what is really happening when reactants such as acids and bases are mixed.
Electrolyte Types
Clearly, if you can identify strong, weak, and non-electrolytes, you are well on your way to identifying the principal species in solution:
| Electrolyte Type | Principal Species in Solution | Example |
|---|---|---|
| Strong | dissociated ions | H+ (or H3O+) and Cl– |
| Weak | intact molecules | HF |
| Non- | intact molecules | O2 |
Notice that it is only the strong electrolytes that principally dissociate into ions. All other compounds mostly stay intact or, in some case, react with water.
Solvation by Water
When any strong electrolyte dissolves in water, the ions that form "react" with H2O to form "hydrated ion complexes." For example, there is evidence that a bare proton, H+, reacts with water to form many species, including (among others) H3O+, H(H2O)2+, H(H2O)3+, and H(H2O)4+. Evidence suggests that Mg2+ is complexed with anywhere from four to six H2O molecules. It is customary to leave these "waters of hydration" out of the net ionic equations. Mostly this is because we can't really be certain in any given case how many waters to include. However, it is also quite common to write H3O+ instead of just H+, as this serves as a reminder that "H+" in water doesn't really exist.
Reaction with Water
There is really no way with the systematic approach we are using to predict accurately what will happen in all cases when substances dissolve in water. Especially for salts, reactions can occur between the cation and water, the anion and water, or perhaps even between the cation and the anion themselves. For this quiz, the cations used for principal species problems are limited to a smaller set referred to as the "Top Ten Cations". (It's a "must-know" list.) You cannot be expected to know more than a handful of these reactions. Nonetheless, here are a few examples of anion reactions with water:
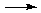 H2(g) + OH–
H2(g) + OH–
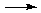 C2H2(g) + 2OH–
C2H2(g) + 2OH–
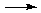 NH3 + 3OH–
NH3 + 3OH–
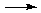 2OH–
2OH–
Note that in all of these cases OH– is one of the principal product, because all of these anions are extremely strong bases able to react completely (and violently!) with water.
Summary
In summary, to make a stab at the principal species in solution, first determine what sort of electrolyte you are working with. Only strong electrolytes (strong acids, strong bases, and salts) principally dissociate into ions. The others stay mostly or completely intact. You will use this information as the starting point for understanding equilibrium reactions.