The name of the synthesized
compound is (+)-Polyoxin J
2. Ghosj, A. and Y. Wang: Total
Synthesis of (+)-Polyoxin J. Journal
of Organic Chemistry 1999,
64:2789-2795.
3. This article was reviewed by Jenny Cho, Jessica Thomes and
Patrick Lytle.
4. The structure of polyoxin J.
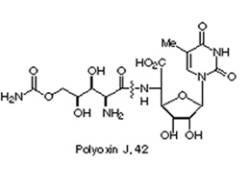
Polyoxin J is a peptidal
nucleotide antibiotics of interest of as an inhibitor of chitin biosynthesis
(Merino, Pedro and Thomas Tejero, 1998).
It is isolated from the culture broth of Streptomyces cacoi, and prevents chitin synthesis from the human
pathogen, Candida albicans which
causes thrush (http://alces.med.umn.edu/candida/Review.html).
6. The retrosynthetic formula, including the Sharpless epoxidation(5,6) is as follows.
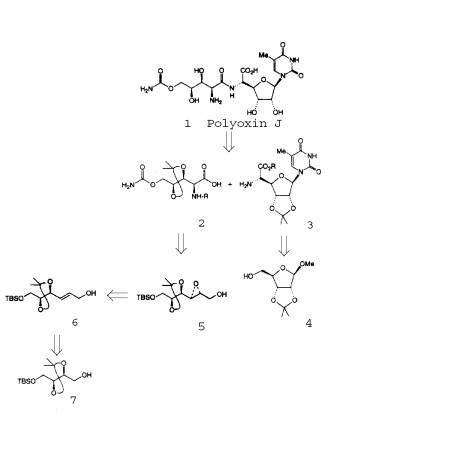
7. The retrosynthetic formula begins with Polyoxin-J, which is
formed by the coupling of a protected O-carbamoyl polyoxamic acid and a thymine
polyoxin C. This step follows a removal
of the protected groups. The protected
groups are the polyoximic acid and thymine polyoxin C. These protected groups are synthesized
stereoselectively from a protected tartrate derivative and methyl glycoside
which are the starting materials.
In order to synthesize the protected 5-O-carbamoyl
polyoximic acid, the researchers introduced an allylic alcohol, derived from
the protected dimethyl L-tartrate. The
exposure of the allylic alcohol to the Sharpless asymmetric epoxidation
conditions with the diethyl D-tartrate molecule yielded a 77% anti-epoxide product.
Silica gel chromatography was used to separate the
enantiomers to obtain the anti product.
A comparable epoxidation, one using m-CPBA
at zero degrees Celsius gave only a 65:35 mixture of anti/syn diasteromers.
Therefore, the Sharpless asymmetric epoxidation proves to be the preferred
reaction.
A regioselective ring opening of the epoxide using
Sharpless’s techniques yielded two diols that are not included in our
retrosynthetic formula. The products
obtained from the ring opening are azido diols. The ingredients for such a ring opening include
diisopropoxytitanium diazide in benzene at 72 degrees Celsius.
The overall goal of this lab was to obtain
(+)-polyoxin J as our major product, and to utilize the Sharpless epoxidation
mechanism so that we can obtain the correct configuration (anti)for
synthesis.
We all shared the tasks of
reviewing this article equally. We all
worked on looking through articles to find the natural source of our compound,
and on attatching the images to our document.
Separate roles included Jenny Cho finding the article, Patrick Lytle
manipulating the images and Jessica Thomes analyzing the text and
molecules. We typed it together and pieced
the fragments together to form the final review of our article.
Jenny Cho
cho@stolaf.edu
Patrick Lytle
lytle@stolaf.edu
Jessica Thomes thomesj@stolaf.edu