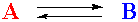
Consider a simple chemical system including just two compounds, A and B:
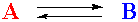
This just says that if you mix A and B, A will react to become B, and B will react to become A. This equation also states that one molecule of A reacts to give one molecule of B. This will be an important part of the concept. A is just turning into B, and vice versa. No other substances are involved (presumably). Associated with this system are two quantities, Q, the reaction quotient, and K, the equilibrium constant.
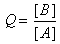
| and | 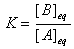
|
It is important to understand the distinction between Q and K.
Q is a quantity that changes as a reaction system approaches equilibrium.
K is the numerical value of Q at the "end" of the reaction, when equilibrium is reached.