(-)
Laulimalide
Paterson, Ian, Chris De Savi, and Matthew Tudge.† 2001.† Total Synthesis of the
††††††††††† Microtubule-Stabilizing Agent (-)
Laulimalide.† Organic Letters 3 (20):
††††††††††† 3149-3152.†
http://pubs.acs.org/isubscribe/journals/orlef7/3/i20/pdf/o101050u.pdf†
Reviewed by Matt Boehm
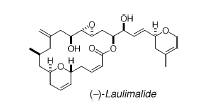
††††††††††††††† Laulimalide is also known as fijianolide
B.† It was isolated from the Pacific
sponges Hyatella sp., Spongia mycofijiensis, and the Okinawan
sponge Fasciospongia rimosa.†
Laulimalide is an anticancer agent.†
It works within the cell cycle by initiating mitotic arrest, micronuclei
formation, and apoptosis.† Laulimalide
has been shown to inhibit the proliferation of numerous cancer cell lines and
appears to have the same microtubule-stabilizing mechanism action as the
anticancer drug Taxol.† In fact
laulimalide was found to be superior to Taxol in its ability to circumvent
P-glycoprotein mediated drug resistance and in stimulating tubulin
polymerization.† It is believed that
laulimalide will be able to provide therapeutic utility against multi-drug
resistant cancers.
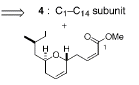
Retrosynthesis
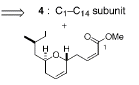
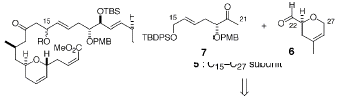
The synthesis of the laulimalide was completed in 27
steps and resulted in a 2.9% yield.† To
start the synthesis, compound 6 is created by a Jacobsenís Diels-Alder reaction
between compound 8 and compound 9.† This
reaction involves the use of a chromium(III) Lewis acid catalyst, which has two
chirality centers.† This allows for the
production of 95% enantiopurity and gave compound 6 one chirality center.† This produces a 6 membered ring and through
a series of steps the methoxy group is removed and the alcohol under goes a
Swern oxidation to remove the H, and produce the aldehyde of 6.† Compound 7 is created from a diol.† Compounds 6 and 7 then undergo a aldol
coupling reaction between carbon 21 and carbon 22 that produces H2O
and compound 5.† 5 then reacts with 4 in
a aldol coupling reaction between carbon 14 of 4 and carbon 15 of 5.† This reaction produces a mixture of
enantiomers.† Then through a complicated
series of steps that include the transormation of a carbonyl group on Carbon 13
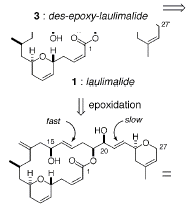
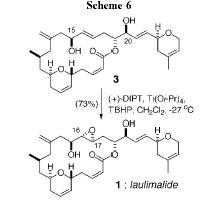
into a carbon-carbon double bond to produce compound 3.† Compound 3 then undergoes a Sharpless
asymmetric epoxidation reaction to produce the final product laulimalide.
The asymmetric epoxidation reaction was a critical
step.† Laulimalide contains an epoxide
between the 16th and 17th carbon.† The Sharpless asymmetric epoxidation allows
for the controled introduction of the epoxide onto the unsaturated diol 3.† The Sharpless epoxidation also allows for
high enantiopurity, which is needed to make laulimalide.† In the synthesis the Sharpless epoxidation
is a very important step, which allows for the proper synthesis of laulimalide.
This
review of laulimalide was put together by Matt Boehm
†††††††††††
†††††††††††
†