The questions asked on this quiz are of five basic forms:
It is very important for chemists to be able to answer these questions with confidence. Obviously the first two are important for communicating in the language of chemistry. But that's not all. The names of compounds are derived from the names of elements and ions. We need to be able to identify compounds by their type so as to have some idea of what their properties are. Names give us a big clue to properties, because the system that has been developed for naming compounds is based on the type of compound being named.
Naming compounds and identifying types is a lot like checkers: The rules are simple, but being successful takes lots of practice. Don't expect to get this down pat overnight, and whatever you do don't expect to be able to cram this for an exam. This quiz can help you sharpen your skills and gain the confidence you need to perform well on your exam. Pick a category such as binary molecular compounds or strong acids, study from your book for 30 minutes, then fire up this quiz and see how well you do. If you do this every day for a week or two, you'll be amazed how much you know.
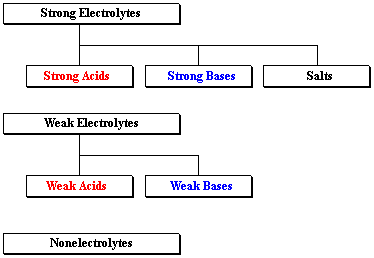
A convenient categorization of compounds is based on the extent to which they become separated (+) and (–) ions when they dissolve in water. To a certain extent, the way we name a compound depends on the type of electrolyte it is.

Strong electrolytes separate 100% into ions when they dissolve in water. They include strong acids, strong bases, and salts. Strong acids and strong bases are easy to identify. (There are only 7 strong acids and about 5 common strong bases.) Salts are a bit trickier to name, but still easy to identify, as all contain metals as their (generally) first-listed element.
| The Seven Strong Acids | Five Strong Bases |
|---|---|
|
|
| Examples of Salts |
|
Notice in these examples how similar the names are within each category.
Weak acids and weak bases comprise the class weak electrolytes, which do not fully dissociate in water. In fact, these compounds are often only 1–5% dissociated. Principally they are in the form written when in water. Weak acid formulas usually start with H, and weak base formulas always contain N. (Although not all compounds containing nitrogen are weak bases, all weak bases contain nitrogen.) It's tricky. To be honest, most practicing chemists just look these things up on tables!
| Six Common Weak Acids | Three Common Weak Bases |
|---|---|
|
|
Notice that weak acids and strong acids are named similarly, so just knowing the name of an acid won't tell us if it is weak or strong. However, weak bases have a whole different--and at this stage in your understanding, probably irrational--naming system. Fortunately, you probably won't need to know more than two or three names of weak bases. (Notice, though that there is "on" or "in" in the weak base names, and there is a nitrogen atom in their formula. This is no coincidence.)
The last category, nonelectrolytes, is huge, containing several million known (i.e. studied and named) substances. Some very important and common nonelectrolytes are binary molecular compounds, which have a simple naming system all their own. These include such environmentally important compounds as water, methane, carbon dioxide, carbon monoxide, sulfur dioxide, and most other components of air.
What's in a name? Well, the name of a compound can give you some clue as to how it reacts, at least what happens when it dissolves in water. If you remember that by learning how to name chemical compounds you are also learning hints about how they react, then the task of learning names may be easier for you.
YOU CAN DO IT!