Chemistry 123 at St. Olaf College.
|
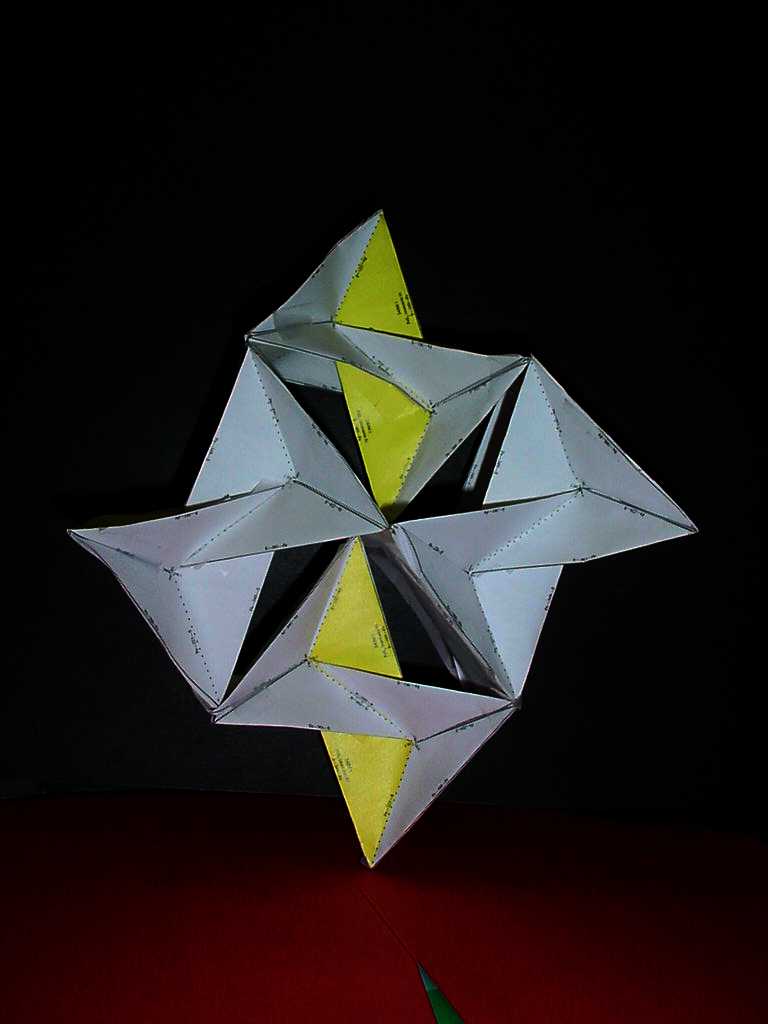
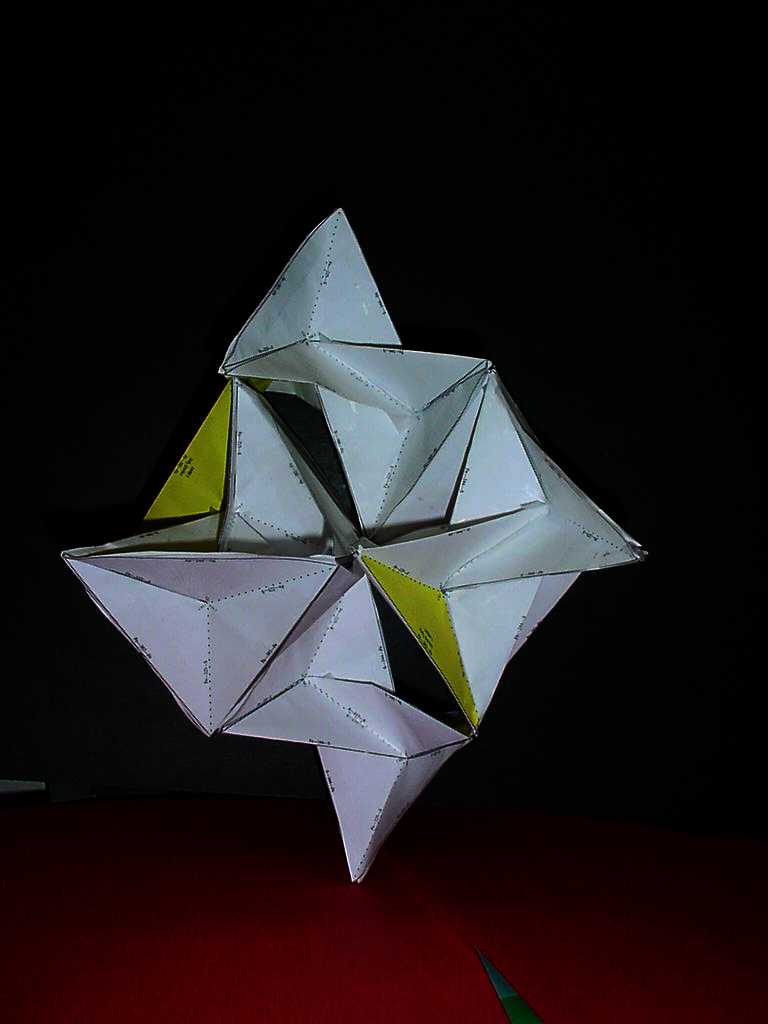
(Go ahead and rotate the model. The top view is what you usually see in books.) The quartz structure consists of SiO4 tetrahedra connected only at their vertices (the oxygen atoms). Notice the right-hand helical twist that gives quartz its natural optical activity repeats every three units. | 
|
| 240,000,000:1 Quartz Model: Unit A Unit B Unit C |
 | 
| ||
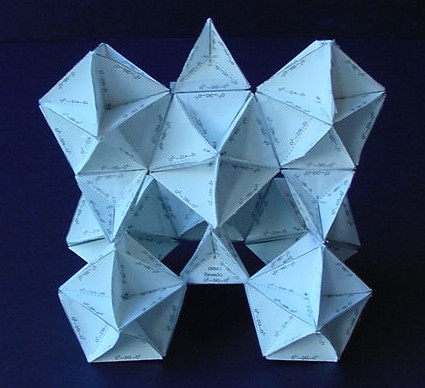
| 240,000,000:1 Marcasite Model: Unit I Unit II |
240,000,000:1 Pyrite Model: units I,II
|
| Both marcasite and pyrite are FeS2. | |
|
Click to see the Zircon (artificial diamond) consists of ZrO8 triangulated dodecahedra linked along two opposite edges by SiO4 tetrahedra. In addition, each ZrO8 unit is connected to four others, two in each of two perpendicular planes.
180,000,000:1 Zircon Model: ZrO8 Unit A ZrO8 Unit B SiO4 Unit |
|
Nitrogenase catalyzes nitrogen fixation in anaerobic bacteria. This active site (molybdenum at the top, iron at the bottom, and six additional iron atoms) is believed to be the site where N2 complexation occurs. | 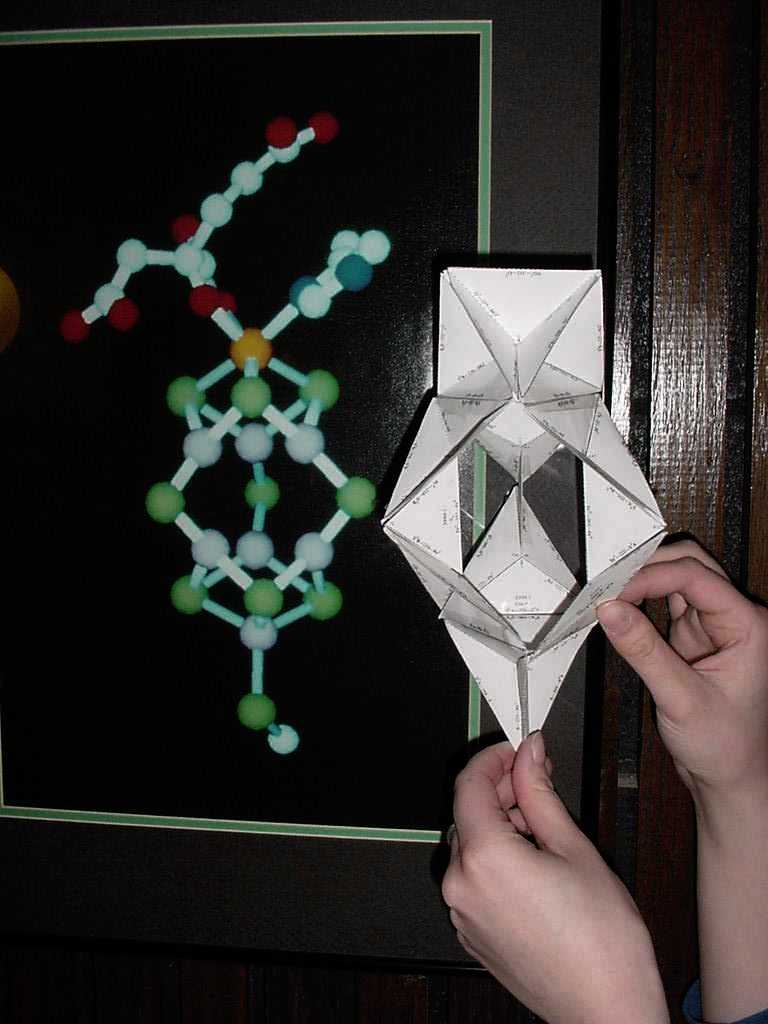
|
| 200,000,000:1 Nitrogenase Model: Molybdenum Unit A Molybdenum Unit B Terminal Iron Fe 2 Fe 3 Fe 4 Fe 5 Fe 6 Fe 7 | |