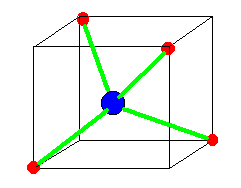
Tetrahedral molecules are under the category, AX4 and have sp3 sigma
hybridization. Methane, or CH4, is a good example of a tetrahedral
molecule. It has four C-H bonds with no lone pairs on any atom. It is
described as a perfect tetrahedron because all of its angles are 109.45
degrees and the bonds are all 1.09 Angstroms. Carbon tetrafluoride, or
CF4 is also a perfect tetrahedral molecule. It has four C-F bonds, with
C as the central atom. The four F atoms have 3 lone pairs on each. The
length of the F-C bonds are all 1.32 Angstroms forming equal 109.45 degree
angles. Sulfuric acid or H2SO4 is also a tetrahedral molecule. Sulfuric
acid has sulfur as its central atom, and has two O atoms, and two OH
groups surrounding it. The HO-S-OH angle is smaller than the O=S=O
angle. This is because the double bonds between O and S create a shorter
distance and the OH groups are repelled by the O atoms coming closer. As
a result, the O=S=O angle is larger than the HO-S-OH angle.
Back to Main Page