Chemistry 247B: Organic
Chemistry Extra Credit Opportunity
1. Name of the compound: 11, 12-epoxycembrene-C
3. Reviewed by: Alex Garton, Tobias Gopon, and Eric Steege
4. Structure of the
compound:
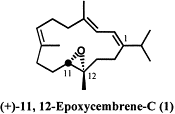
5. Natural occurrence and
medicinal purposes:
Cembrene epoxides, such as (+)-11,
12-Epoxycembrene-C, have been discovered in many various marine soft corals.
Our substance was first isolated from an Australian soft coral, Sinularia grayi, in 1978 by Bowden and
his co-workers. After this first isolation, the compound was found in other
soft coral as well, including Nephthea,
Lobophytum, and Eunicea. Even though in the article it says that our compound has
many “biological activities,” we were unable to find a medicinal purpose.
Although in a meeting with Charlie Priore, the science librarian, we were able
to find the registry number of our compound (69778-94-5) and searched numerous
databases including SciFinder Scholar, Web of Science, Cambridge Abstracts, and
ACS Publications, we were unable to find a positive lead to any medical uses.
To show the difficulty of finding any medical reference to this compound, the
author of this synthesis, or even this type of compound, we searched the Web of
Science for Yulin Li, the author, and exactly 2174 hits came up. After
searching the first 500 articles, only one of those was in a medical journal,
the Journal of Medicinal Chemistry, and the article was unrelated to our
compound and its class.
 6. Retrosynthesis (including Sharpless asymmetric epoxidation):
6. Retrosynthesis (including Sharpless asymmetric epoxidation):
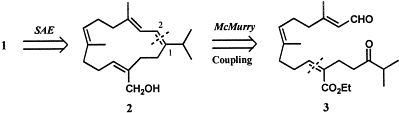
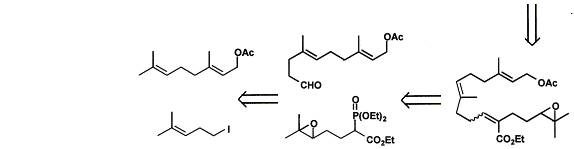
7. The overall idea of this retrosynthesis was to use effective reactions, producing high yields, to make a natural occurring compound. This retrosynthesis highlights the two main components of producing this compound and how to get to those two steps from the initial reactants. The initial reactants were made into compounds by “standard method[s]” that could be connected together (addition of CHO and P(OEt)2=O). Those two compounds were then connected using the Wadsworth-Horner-Emmons coupling in LDA. Then, the united compound was readied for the McMurry coupling (making of =O from epoxide and substitution of OAc with CHO). The McMurry coupling was used because it is a “valuable and versatile protocol for the construction of [a] carbocyclic skeleton.” This joined the two ends of the compound together to form a 14-membered ring. Then, the last step, the Sharpless asymmetric epoxidation, was done to form the crucial epoxide of the epoxycembrene because it produced a high yield typical of this specific epoxidation and it is stereospecific producing the identical stereochemical configuration of the natural occurring compound.
8. Team work
distribution:
Each of the members of our team played an equally important role to the success of this project. From the time we started by selecting an appropriate journal article to the finalization of the paper, we all researched and brainstormed together in order to fulfill all the necessary requirements together as a team.