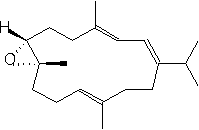
(-)-7,8-epoxy-7,8-dihydrocembrene-C
First enantioselective
total synthesis of (natural) (+)-11,12-epoxy-11,12-dihydrocembrene-C and
(-)-7,8-epoxy-7,8-dihydrocembrene-C. Liu, Zuosheng; Li, Weidong Z.; Peng, Lizeng; Li, Ying; Li,
Yulin. National Laboratory of Applied
Organic Chemistry and Institute of Organic Chemistry, Lanzhou University,
Lanzhou, Peop. Rep. China. Perkin 1
(2000), (24), 4250-4257.
CODEN: PERKF9
ISSN: 1470-4358. Journal written in English. CAN 134:222884 AN 2000:887392
CAPLUS
Reviewed By:
Margaret Currie currie@stolaf.edu
Joel Tombisammy tombisam@stolaf.edu
Christopher Sumey sumey@stolaf.edu
This compound comes from a soft coral organism called Sarcophyton crassocaule and also from Eunicea. These coral are most commonly located in the oceans near the Philippines and Fiji Islands. The chief use of this compound is for fighting some cell lines of cancer.
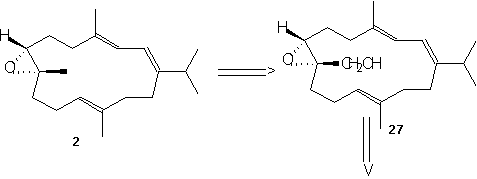
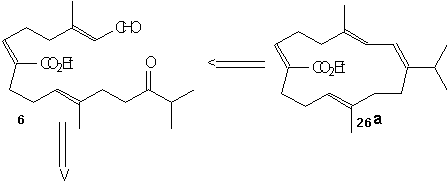
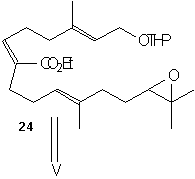
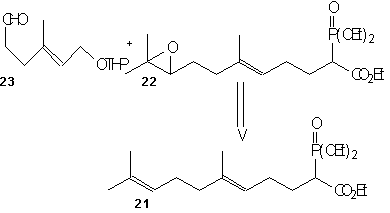
(-)-7,8-epoxy-7,8-dihydrocembrene-C 2 is formed directly from iodination and then dehalogenation of epoxide 27. This epoxide 27 can be produced by Sharpless asymmetric epoxidation of ester 26a using Ti(O1Pr)4, L-(+)-DET, and t-BuOOH at –20°C. This ester was formed by cyclizing 6 using TiCl4, Zn, and THF and refluxing to give the desired Z-conformation. Keto aldehyde 6 was formed by treating 24 with LiClO4 in bezene, then using acidic hydrolysis and MnO2 oxidation. Ester 24 was produced by coupling 22 with the aldehyde 23 using the base Lithium diisopropylamide (LDA). LDA is a very strong base however it acts as a poor nucleophile which allows it to remove the alpha-hydrogen much quicker and allows the aldehyde (22) to easily couple to the reagent (23). Phosphono ester 21 underwent regioselective epoxide formation through a bromohydrin intermediate to give 22. This was done using NBS, K2CO3, and MeOH. This reaction appears to be a substitution reaction because of the replacement of a double bond with an epoixide at extremely low temperatures (0˚ C) is an example of a partial Sn1/Sn2 reaction. Secondly we could make a case that this reaction is influence by Sn1 for two reasons; first, because of large size of the molecule a Sn2 reaction would be difficult because of the amount of steric hindrance. Second, the presence of MeOH signals us to a Sn1 reaction because it is a protic polar solvent, and we know that Sn1 reactions thrive in conditions of polar protic solvents. Ester 21 can be made using its corresponding homogeranyl iodide.
The Sharpless epoxidation reaction was used in order to create this compound because it allows the scientist to control the stereochemistry of the product. Despite starting with an enantiomerically pure epoxide, with out employing the Sharpless epoxidation reaction, it is entirely possible to end up with compounds with a mix of different chirality centers. This is a very valuable tool because it allows us to discriminate between two very similar compounds.
Contributions:
Joel Tombisammy assisted in analyzing the reactions and provided clarifications of the reactions caused by particular solvents. He and Margaret found the article and initial analysis of the reaction. Margaret Currie created the wonderful retrosynthetic diagrams.
Christopher Sumey contributed the medicinal uses of the compound, as well as analysis of the conditions of each step.