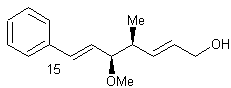
(+) – Crocacin
C
Chakraborty TK, Jayaprakash S,
Laxman P. Total synthesis of (+)-crocacin C. Tetrahedron 2001;57(46):9461-9467.
http://www.sciencedirect.com/science/article/B6THR-44C8T5Y-F/1/1bda2a711363a1360e9974d68e11a6fd
Reviewed by:
Erin E. Albers, Timothy P. De Chant, and Abby M. Sprenger
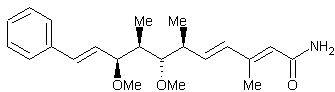
(+) – crocacin C
The natural source of the
compound (+) – crocacin C is Chrondromyces
crocatus. Chrondromyces crocatus is a type of bacteria which acts as a
fungus, it comes from a species of Myxobacteriales. (+) – crocacin C is of interest to makers of medicine because it
can be used as a antifungal or cytotoxic agent. The antifungal part applies to any sort of fungus, be it athletes
foot, or what have you. (+) – crocacin
C is also a cytotoxic agent. By
Webster’s definition a cytotoxic agent is one “that harms or destroys living
cells (cytotoxic drug therapy), or relating to cytotoxins, their effects.”
(Webster’s Dictionary). A cytotoxin is, therefore, “ a chemical substance that
destroys, or impairs the function of, specific living cells.” This would be very useful in the treatment
of many forms of cancer because it only destroys specific cells. If it was possible for the cytotoxins to
attack the cancer cells we might be on our way to finding the cure for
cancer. This could also be a reason why
we only need one diastereomer of the molecule, for if we had more then one the
resulting effect could either be a cancellation of the desired effect or a
potentially harmful one.
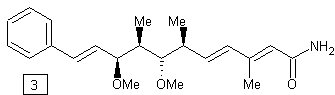
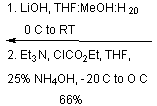
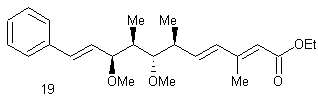
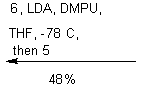
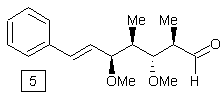
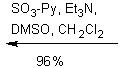
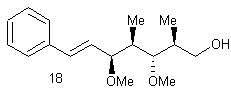
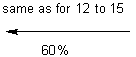
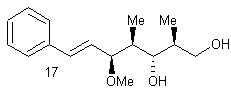
![]()
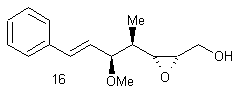

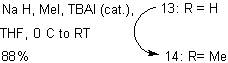
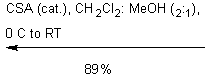
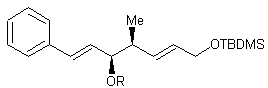
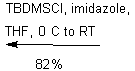
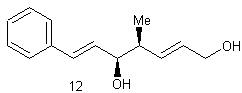

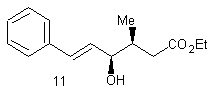
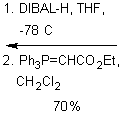
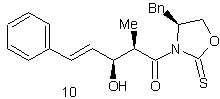
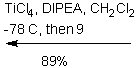
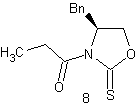
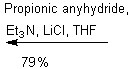
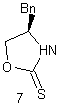
The purpose of the
Sharpless asymmetric epoxidation ensured both the formation of the desired
epoxy alcohol and a high yield of that desired compound (93% in this
case). The high yield in this case was
extremely advantageous, saving the chemist both time and money by making the
most of the available compounds. By
almost guaranteeing a diastereometrically pure sample, the chemists were better
prepared for the next step of their reaction, the regioselective opening of the
epoxide.
For this project Erin drew
all of the molecules, looked up information and helped to look for an
article. Timothy worked on the
discussion of the retrosynthesis and revising the paper. Abby found the article, did some research,
and typed the paper.