Reviewed by: Kristi Parks, John Grau, Kristi Hembre
Review of Antitumor Depsipeptide (-)-Doliculide
http://pubs.acs.org/isubscribe/journals/orlef7/3/i04/pdf/ol0100069.pdf
Ghosh, A.K. and C. Liu. January 12, 2001 Vol
“Total Synthesis of Antitumor Depsipeptide (-)-Doliculide” Organic Letters. Department of Chemistry, University of Illinois at Chicago. Vol.3 No.4. 635-638.
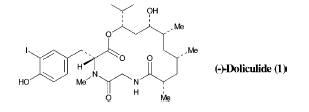
(-)-Doliculide is a compound, a depsipeptide that is a carbonyl and methylene alternating compound, which contain ester bonds and peptides, derived from the Japenese sea hare, Dolabella auricularia. It has been used as a toxic agent against HeLa-S3 cells, laboratory cells which are a virulent strain of cancer cells that reproduce in laboratory setting. They have been one of the main cells used in cancer research because they are so virulent and have quick reproduction rate in vitro. This structure was also determined using NMR studies. (-)-Doliculide inhibits growth of cancer cell lines. Other chemicals extracted from the sea hare, which are similar to the (-)-Doliculide, are presently being used in clinical trials for treatment of cancer by interacting with tubulin to induce apoptosis, which is programed cell death.
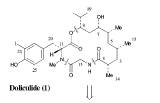
The strategy for constructing the retrosynthesis of (-)-Doliculide depends on the synthesis of polyketide unit 2 and synthesis of D-tyrosine derivative 3, and its addition to a tyrosine-glycine dipeptide, and then assembly of the molecule by an esterification, which is a carboxyl group and methyl alcohol forming a methyl acetate, and cycloamidation reaction sequence. Cyclyoamidation is a reaction which forms an amid as well as an aromatic ring. A polyketide is a benzene ring with alternating carbonyl and methyl groups.
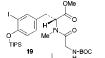
Molecule 17 is transformed into Tyr-Gly Dipeptide, molecule 19. The epoxide steps in the retrosynthesis shown below will lead to the formation of molecule 16, which is added to molecule 19 to make a molecule when transformed is the (-)-Doliculide molecule.
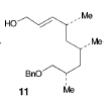
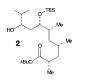
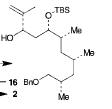
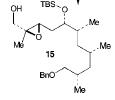
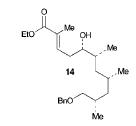
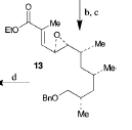
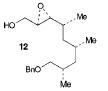
The retrosynthesis of the epoxide steps are the following: Molecule 16 results from a diol transformed to tert-butyl ester in a three-step sequence. In order for this to happen 16 is hydrogenized by debenzylation and saturation of olefin, an alkene which makes an allylic alcohol. In order for this to occur molecule 15 must be converted to its iodide by mesylation and displacement of mesylate that is an Sn2 reaction. Epoxy molecule 15 was made from sharpless epoxidation of (+)-DET, which is going from double bond to an epoxide. Anti alcohol 14 was formed by the addition of n-Bu3P and catalytic amount of Pd2(dba)3 and CHCl3 in HCOOH-Net3 after the regioselective epoxide opening, forming an epoxide alcohol, 13 remains a mixture. Molecule 13 comes from sharpless epoxidation. Molecule 13 is an E/Z mixture after a reflux in benzene. The Wittig homologation, uses phosphorus to preform a homologation, of aldehyde and ylide Ph3P+C(CH3)CO2C2CH5, which is a compound with opposite charges on adjacent covalently bonded atoms with complete octets of valence electrons. This will then go through Swern oxidation. The epoxide 12 and its diastereomer are isolated as a mixture, which is the cause of the lower diastereoselectivity. Molecule 12 is formed by the allylic alcohol 11 undergoing sharpless epoxidation with (-)-DET.
The overall idea was to stereoselectively synthesize a polyketide unit and the D-tyrosine derivative. To due this they installed a 1,2-dimethyl group and a 1,3 diol unit which undergo the asymmetric cyclopropanations and the sharpless epoxidations as key steps. Note that the sharpless epoxidation is carried out more than once. The researchers used the asymmetric epoxidation reaction to construct the 1,3 diol unit and to form epoxid 12 and its diastereomer in 90% yield so they could work with it.
Kristi Parks worked on finding information about the medical usages of the (-)-Doliculide and the natural sources from which it was derived. She also had a good understanding of the reaction as a whole and helped map out the diagrams of the retrosynthesis. John Grau worked on the highlighted usage of the sharpless epoxidation, the overall idea, and why the researchers used the sharpless epoxidation in the synthesis. He also helped the group to better understand the overall necessary steps involved. Kristi Hembre worked on the retrosynthesis of the epoxidation steps and the overall synthesis of the molecule.
![]() +
+ 
![]()
![]()
![]()
![]()
![]()
![]()
![]()