Total Synthesis of (-)-Dysiherbaine
Hidekazu Masaki, Junji Maeyama, Kazuko Kamada, Tomoyuki Esumi, Yoshiharu Iwabuchi, and Susumi Hatakeyama.† Journal of American Chemical Society. 2000, 122, 5216-5217.
Reviewed by: Melissa Kimmerling and Janet Rasmussen
Background:
Dysiherbaine is a neuroexitatory amino acid isolated from the Micronesian sponge Dysidea herbacea that causes seizures upon injection into mice.† It is a selective agonist of non-N-methyl-D-aspartate type glutamate receptors that may help in evaluating the physiological and pathological roles of non-NMDA receptors in the central nervous system (Masaki 2000).† A study evaluating the interactions between the neurotransmitter L-glutamate and the proteins it binds to during neurotransmission uses the dysiherbaine to identify its selectivity and potency when binding to the receptors and glutamate system.† This could lead to a better understanding of processes such as learning and memory, and new details on mechanisms of brain damage in disorders such as strokes, hypoglycemia, epilepsy, Huntingtonís disease, ALS, and Alzheimerís disease (www.chem.uci.due/people/faculty/archambe).
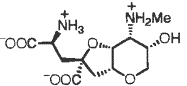
Retrosynthesis:

The asymmetric epoxidation was used in the synthesis for the conversion of σ-symmetrical divinylcarbinol to epoxy alcohol.† This method was used because it allows for a specific stereoselectivity, so that the identical product can be formed in terms of spectroscopic and chromatographic comparisons.† The final product of (-)-dysiherbaine in figure 1 has six chirality centers, three of which (a, b, and c) are a direct result of the stereoselectivity formed by the epoxidation process that forms figure 7 from figure 6.† Chirality centers a and c retain the same stereoselectivity through the synthesis with the oxygen, while the stereoselectivity of b in 7 allows for the nitrogenís stereoselectivity to fit that of the final product.
Acknowledgments: Both reviewers contributed equal amounts of time to this review, as Melissa Kimmerling concentrated on the potential uses of this compound while Janet Rasmussen concentrated on the retrosynthesis.