Asymmetric Epoxidation in the New Millenium
Extra Credit, Organic Chemistry 247B
Ann Musgjerd, Megan Thorvilson, and Kathy Van Heuvelen
- (-)-Isolaurallene
- M. T. Crimminsand, K. A. Emmitte. “Asymmetric Total Synthesis of (-)- Isolaurallene.” Journal of the American Chemical Society, 2001. 123: 1533-1534.
http://pubs.acs.org/CHECKCCIP1007947293/isubscribe/journals/jacsat/jtext.cgi?j acsat/123/7/html/ja005892h.html
- Reviewed by Ann Musgjerd, Megan Thorvilson, and Kathy Van Heuvelen
-
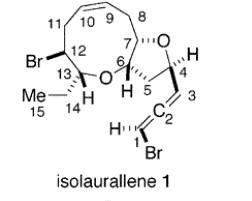
- Marine organisms are the natural source of this compound. This compound has been isolated by Kurata on the Pacific Coast of Hokkaido in Izumihama near Hiroo. Medium ring ethers, such as isolaurallene, have been found to impact many biological processes.
-
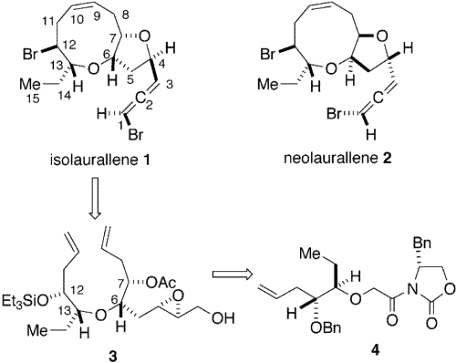
- In this synthesis, a nine-membered ring ether was produced from oxazolidinone using a ring-closing reaction. To avoid the use of a cyclic conformational constraint, the researchers used the rapid and stereocontrolled ring-closing metathesis. The original reactant contained a five-membered ring, which was opened using NaBH4, H2O, and THF. The chain was enlarged throughout the reaction and, immediately after the first epoxidation reaction, the chain was exposed to the Grubbs catalyst to form the nine-membered ring. The synthesis includes two Sharpless epoxidation reactions, each of which produces only the desired product instead of a mixture of isomers.
- This project was a group effort. We all met to find an appropriate article. After the article was approved, we met again to discuss the synthesis and write the review. We worked well together; everyone contributed equally.