Chiral Juvenile Hormone I
Prestwich, G.D. & Wawrzencyk, C. 1985. High specific activity enaniomerically enriched juvenile hormones: Synthesis and binding assay. Proc. Natl. Acad. Sci. USA. Vol. 82, 5290-5294.
Reviewed by: Cathy Hovde
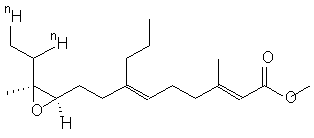
Juvenile hormone I, as the authors refer to it, is found primarily in insects. Normally though, it is synthesized for experiments dealing with a putative low-abundance high-affinity hormone. Until now the synthesis procedure has produced a racemic mixture that was inadequate for experiments, possibly leading to competitive displacement of the natural analog or to excessive nonspecific bindings. These limitations were not completely debilitating to researchers but with a >95% enantimerically enriched hormone available, I’m sure experiments will go much more smoothly.
Retrosynthesis:

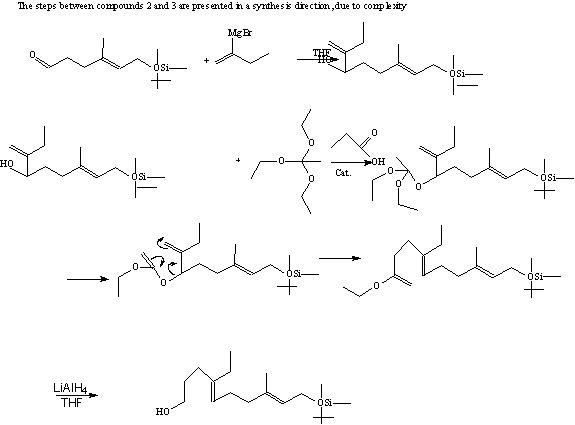
In the retrosynthesis there are several very interesting steps worth highlighting. In the first step is a grignard addition, SN2, to the initial reactant, which uses the Magnesium to make the butyl group partially negative and thus a nucleophile. The next step is an SN1 addition, in the form of a Claisen rearrangement; this uses a semi- diels-alder reaction to accomplish the rearrangement. Finally, to get the final product needed LiAlH4 is added to remove the ester and double bonded Carbon and produce an alcohol. The alcohol is preferable because in the next step it is hydrated and replaced in an SN1 reaction by TsCl. The toluene is preferred to OH because it is a better leaving group, so it can be replaced by Iodide. The whole point of these several reactions, which change the leaving group several times, is to prepare the molecule to have a butyl group and the THPO substituted on to the molecule. In the next set of steps the reaction the OSi is replaced using several steps to substitute the O with the trienal. The MnO2 then oxidizes the trienal. Finally, the molecule is converted to the methyl trienoate with MnO2 in the presence of acetic acid, sodium cyanide, and methanol. Once the THPO is replaced the molecule is ready for the Sharpless epoxidation reaction. This reaction works because the pi bond between the three different carbon groups is able to act as a nucleophile to the O in the t-butylhydroperoxide that is joined to an iso-butyl and alcohol group. The alcohol group is removed because it is good leaving group, as it stable as a base. Since epoxidation works to add to the O on both sides it is able to keep its electrons and release the iso-butyl cation, which is stable due to its branching. The asymmetric epoxidation was used because it produced the needed chiral compound, (10R, 11S)-epoxy alcohol. Once the epoxy is added the last steps of the reaction can take place. The alcohol, which replaced, THPO is then dehydrated to produce a double bond. This pi bond is then hydrogenated with 3H, this allows researches to follow this hormone through the experiment.
Honest
assessment
Seeing how I was the only person in my group, I did most of the work. However, I’d like to thank Prof Bob Hanson for his time and creativity when helping me.