9-methoxystrobilurin K
Uchiro, H., K. Nagasawa, Y.
Aiba, T. Kotake, D. Hasegawa & S. Kobayashi. 2001. Asymmetric total
synthesis of 9-methoxystrobilurin K.
Tetrahedron Letters. 42:4531-4534.
Online
source: ScienceDirect
Reviewed
by Amy Kruchowski and Christina Richter
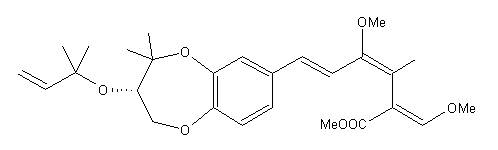
9-methoxystrobilurin
K was originally found in a mycelial culture of forest fungi. It was noted that the isolated substance
exhibited antifungal characteristics to invading organisms by preventing
mitochondrial respiration and killing the cell. Naturally occuring 9-methoxystrobilurin displays characteristics
of beta-methoxyacrylate antibiotics (MOAs) and in small concentrations has been
shown to stop the growth of tumor cells without killing them. Ideally, treatment by such an agent could be
an alternative to chemotherapy for cancer patients. But 9-methoxystrobilurin K would not reduce the size of the tumor
and surgery may still be necessary. It
is not yet known what effects large concentrations of 9-methoxystrobilurin may
have on living cells.
Retrosynthetic
Scheme:
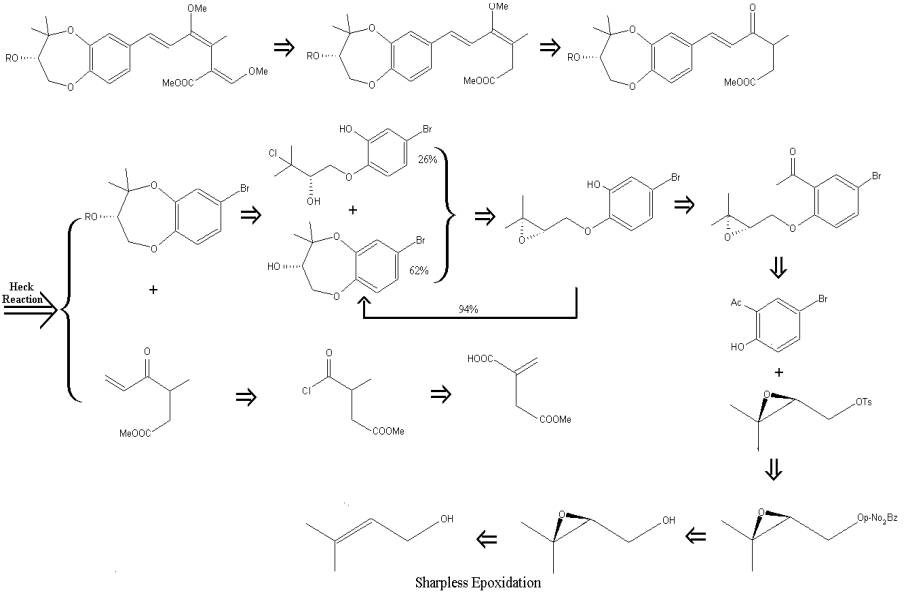
Highlights
of the Retrosynthesis:
The
synthesis was centralized around a Heck reaction, which is the coupling of an
organic bromide and an alkene with the use of a palladium catalyst. The Heck reaction is one method of extending
an organic chain. The first major
reaction in the synthesis of 9-methoxystrobilurin K produced an optically
active chiral epoxy phenol which was the analogue to the desired
7-bromo-1,5-benzodioxepin-3-ol. The
previous bromo alcohol was coupled with an alkene to form an ester. The processes of hydrolyzation and treatment
by a hindered base followed by double methylation produced the desired
9-methoxystrobilurin compound along with two other undesired products. Attempts made to irradiate the final
products yielded a total of 41% 9-methoxystrobilurin.
H.
Uchiro et al. chose to use the Sharpless asymmetric epoxidation reaction to
form the desired chiral epoxy because of the reaction's high yield. The asymmetric epoxidation reaction
successfully converted 85% of the prenyl alcohol to epoxy phenol.
Christina
located the article and typed up the report.
Amy got the report approved, created, and attached all compound
structures and the retrosynthesis. Together,
we edited the report and sought to understand the article, and the purpose of
the complex.