Chem 247 – Synthesis Review
12-11-01
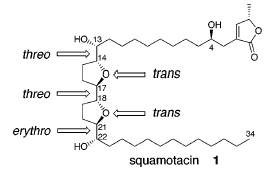
Compound: Squamotacin
Sinha, Santosh C., Subhash C. Sinha, and Ehud Keinan. Total Synthesis of Squamotacin. Journal of Organic Chemistry. 64: 7067-7073. 1999. http://pubs.acs.org/isubscribe/journals/joceah/64/i19/pdf/jo990599p.pdf
Reviewed by: Molly Fee, Jeremy Glen, Amanda Johnson
Background:
Squamotacin is naturally found in the bark of Annona squamosa. It is one of a family of compounds known as Annonaceous acetogenins, many of which are powerful antitumor, antimalarial, and immunosuppressive agents. Squamotacin in particular is an inhibitor of NADH oxidase, a membrane enzyme that is active in tumor cells and inactive in normal cells. Squamotacin also inhibits NADH-ubiquinone, a complex involved in mitochondrial electron transport systems. By inhibiting these cell activites, squamotacin reduces ATP production, which eventually causes programmed cell death. Studies have shown squamotacin to be effective in treating a human prostrate tumor cell line (PC-3).
Retrosynthesis:
The complete synthesis involved 27 steps. The generalized scheme is as follows:
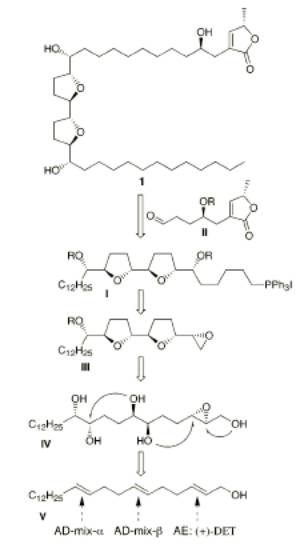
The basic plan of the retrosynthesis was to break squamotacin into two smaller molecules (I and II). Molecule I was synthesized from an epoxide (III, IV), which in turn was synthesized from an alkene (V) via an asymmetric epoxidation reaction and two Sharpless asymmetric dihydroxylation reactions. The asymmetric epoxidation took place on the 2-3 double bond, and the asymmetric dihydroxylation reactions took place on the 6-7 and 10-11 double bonds. Asymmetric dihydroxylation adds an OH group to each side of the double bond.
One advantage of this retrosynthesis is that the epoxide formed (III, IV) is enantiomerically pure. A pure epoxide can be used to synthesize a compound with two adjacent chirality centers. The epoxide is pure because the asymmetric reactions used to create it produce only one enantiomer. In asymmetric epoxidation, the structure of the epoxide depends solely on the stereochemistry of the diene, so a predictable product is synthesized. In asymmetric dihydroxylation, a chiral catalyst is used, so a pure enantiomer is formed for these bonds, too.
Acknowledgements:
All three members of the group contributed equal time to the project. Molly found the article online and highlighted important sections. Amanda and Jeremy wrote up the formal report.