Hemoglobin | |
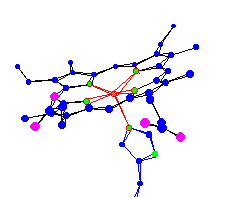 de-oxy hemoglobin |
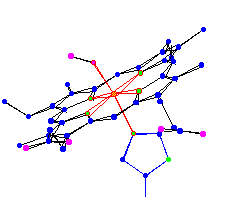 oxy hemoglobin |
| Hemoglobin, used by all vertebrates and many invertebrates,
is the most common of the oxygen transport proteins. It carries dioxygen
(O2) in blood from the lungs to tissue in the rest of the body
to assist in metabolism. |
|
| Notice the difference in position of the Iron (the center
atom) in oxy and deoxy. Theory 1 - The position is not really affected by the presence of O2, but is what determines if an O2 will be able to bond. That is, when the Iron is in the plane of the protoporphyrin IX (often referred to as -heme) it is able to bond with dioxygen, and conversely when the Iron is below the plane, it is impossible for dioxygen to bond or remain bonded. Changes in the amino acid chain or in the environment can cause a change in shape of the heme, which in turn can cause a change in oxygen affinity. For example: in low pH O2-Fe affinity is lowered. When hemoglobin enters the tissues, which have a high concentration of waste carbon dioxide, the oxygen readily disassociates. (Carbon dioxide is an acid (low pH) because it hydrates (combines with water) into H2CO3, which disassociates into HC3 and H+. Changes in the actual chain sequence can effect the oxygen affinity as well. The solubility of hemoglobin is lowered by 100 times when a valine is substituted for specific glutamic acid. This condition is referred to as sickle cell aminia. Theory 2 - The iron is too large to fit in the middle so it is below the molecule about 0.4 angstroms. But when it is bonded to dioxygen one of the valance electrons becomes used by the Fe-O bond. In theory this lowers the radius enough to let the Iron atom fit in the plane of the ring. For thought - Carbon Monoxide (CO) bonds to hemoglobin 210 times stronger than Dioxygen (O2). Why might this occur? |
|
Hemerythrin | |
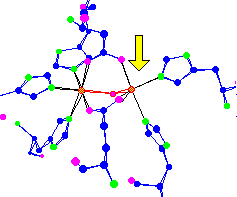 de-oxy hemerythrin |
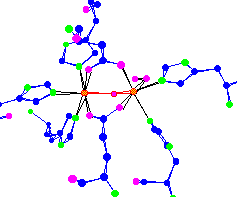 oxy hemerythrin |
|
Hemerythrin is used by
- worms, cephalopods, crustaceans, and spiders
- sipunculid worms
- four phyla of marine invertebrates
For thought - Unlike hemoglobin, hemerythrin is not poisoned by carbon monoxide (CO). What makes hemerythrin safe from CO poisoning? | |
Hemocyanin | |
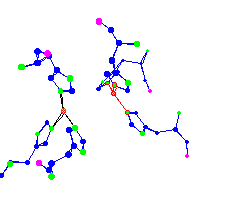 de-oxy hemocyanin |
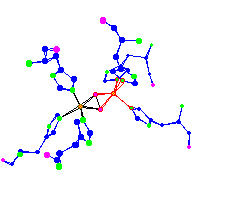 oxy hemocyanin |
|
Haemocyanins are copper-containing oxygen transport proteins found in the
haemolymph of many invertebrates. They are divided into 2 main groups -
arthropodan and molluscan.
The histidines are more planar in the deoxy than in the oxy. This planar shape would make the iron less available to bond with the oxygen in the deoxy form. For thought - Hemocyanin releases oxygen three times easier than hemoglobin. What about the active site bonding, structure of the rest of the protein, or conditions in the tissue that causes that? | |
Photosynthetic Reaction Center | |
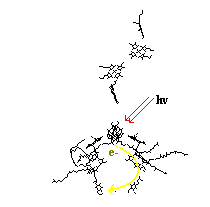
|
|
|
In 1988 Dr. Johann Deisenhofer, Professor Robert Huber, and Dr. Hartmut
Michel won the Nobel Prize in chemistry for their work with
photosynthesis and the photosynthetic reaction center. You can read
about The
1988 Nobel Prize in Chemistry. In the Photosynthetic Reaction Center a large part of the protein is functionally important. (This differs from many other proteins that weve looked at, where most of the protein is not involved in the reaction.) Light (hv -red arrows) comes in and hits a bacteriochlorophyll dimer. This excites an electron up to a higher state, with enough energy to break off. The electron then follows the yellow path around and comes to rest on a quinone. Once the quinone has two electrons, it breaks off. Three electrons are enough to convert ADP to the highly energetic ATP. Once ATP releases its energy the electron makes its way back to the bacteriochlorophyll dimer. For thought - Chemically why and how are the electrons shuttled through the photosynthetic reaction center? |
|
ADP + ATP |
|
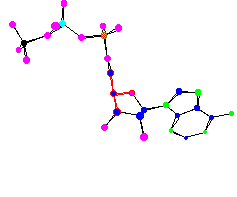 ATP - Adenosine Triphosphate The energy currency of the cell |
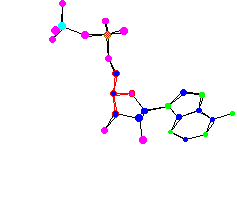 ADP - Adenosine Diphosphate |
| ATP, ADP, and phosphates in the body are involved in a continuous cycle to shuttle energy around to power cells. In ATP all three phosphates have a negative charge and are all in a line right next to each other, which makes for a high energy situation. When one of the high energy bonds in ATP is broken about 7 kcal/mol of energy is released. | |
Back to the Molecular Origami Home Page