Trigonal Pyramidal Zinc Center
One of the possible geometries for zinc in proteins is trigonal pyramidal. Trigonal pyramidal geometry occurs when a central atom has three ligands bonded to it so that the four atoms are not in the same plain. The degree to which the four atoms are non-planar varies.
|
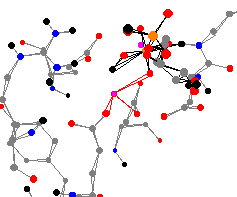
Fructose-1,6-bisphosphatase is a key regulatory enzyme in gluconeogenesis.
This important enzyme catalyzes the hydrolysis of D-fructose-1,6-bisphosphate
to D-fructose-6-phosphate. It is regulated by two inhibitors: AMP and
fructose-2,6-bisphosphate. Fructose-1,6-bisphosphatase is activated by divalent
metal ions in low concentrations.
|
|
High concentrations make it less active.1 This zinc center has three oxygen atoms attached to it from a glutamic acid, an aspartic acid, and 2,5-anhydroglucitol-1,6-biphosphate. The average bond length is 235 pm. This center is less planar than the other two centers on the page, with the average angle at the peak of the pyramid being 101°.
| |
1Zhang, Y., Liang, J., Huang, S., Ke, H., Lipscomb, W. Biochemistry
1996, 32, 1844.
|
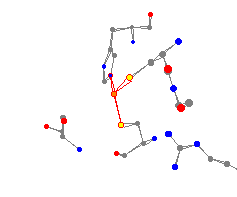
Horse liver alcohol dehydrogenase is an NAD-dependent enzyme. It is a dimer,
and therefore, contains two identical subunits. This enzyme is very versatile.
It works on a large number of alcohols and aldehydes, especially primary and
secondary alcohols. The substrate binds to the enzyme at a hydrophilic pocket with the oxygen becoming a ligand to the zinc.2
|
|
(This situation was not shown in the crystal structure.)
This zinc center is surrounded by two histidine groups (attached through a nitrogen) and a cysteine group (attached through a sulfur). The average bond distance is 220 pm, and the average peak angle is 115°. A planar center would have the peak angles equal to 120°. This is the "flattest" pyramid in this study.
| |
2Ramaswamy, S., Eklund, H., Plapp, V. Biochemistry
1994, 33, 5230.
|
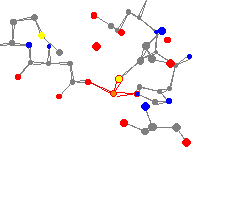
1kev is a tetrameric NADP-dependent secondary alcohol dehydrogenase. The main point of interest for the study of this enzyme was to attempt to investigate structural differences that might account for the ability of certain proteins to withstand more harsh conditions than other proteins.
|
|
One of the samples was taken from a thermophilic baterium, and the other was not.3 This zinc center is attached to a nitrogen, a sulfur, and an oxygen atom. The nitrogen atom is the closest, 191 pm, then the oxygen, 215 pm, and then the sulfur at 230 pm. The average peak angle is 113°.
| |
3Korkhin, Y.,Frolow, F., Bogin, O., Peretz, M., Kalb, A., Burstein, Y. Acta. Cryst. Sect. D 1996, 52, 882.
Tetrahedral Zinc
Trigonal Bipyramidal Zinc
Two Other Zinc Structures
Back to Study Introduction Page
Back to Molecular Origami Home Page


