Bent | |
| Both sulfurs of a (2Fe-2S) Ferredoxin are bent. This also is used for electron transport |
 |
| Bent geometries are really pretty easy to find. One word of caution though, make sure that there would be no hydrogens attached to the center atom. (X-ray diffraction results do not include hydrogens.) | |
Octahedral |
|
| Iron center of hemerythrin An oxygen transport molecule for lower forms of life, worms and molluscs. This iron is always octahedral. |
 |
| Iron Center of Myoglobin Used for oxygen storage in the tissue The protoporphyrin IX is nearly identical to the same in hemoglobin. |
 |
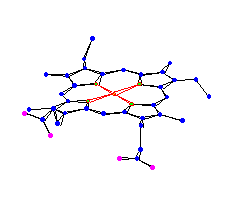
Square Planar |
|
| Protoporphyrin IX from hemoglobin Hemoglobin is used for oxygen transport in humans. This is really an incomplete picture, it has a histidine attached to the top. |
 |
Square Pyramidal |
|
| Protoporphyrin IX and Histidine in hemoglobin Hemoglobin is used for oxygen transport in humans. When it is in this square pyramidal shape it can to pick up an O2 for transport |
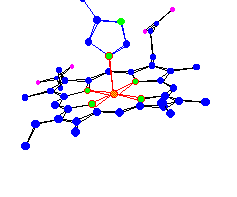 |
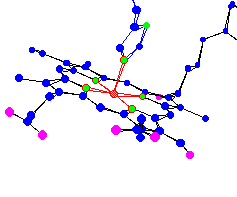
| Heme-A of Cytochrome-c oxidoreductase. Part of a system that converts O2 and H+ into H2O in the cell membrane |
 |
Tetrahedral |
|
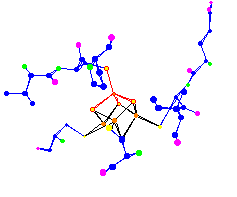
| The 4 Iron (Fe) Centers of a (4Fe-4S) Ferredoxin. Used for electron transport. 1blu |
 |
| The 2 Iron Centers of a (2Fe-2S) Ferredoxin Also used for electron transport | 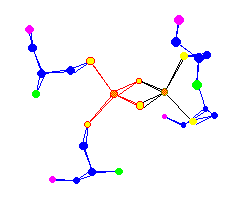 |
| Phosphorus surrounded by 4 oxygen molecules Part of a DNA chain |
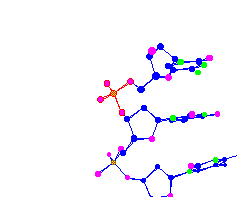 |
Trigonal bipyramidal |
|
| Iron center of hemerythrin An oxygen transport molecule for lower forms of life, worms and mollusks. This Iron can become octahedral and pick up an O2 |
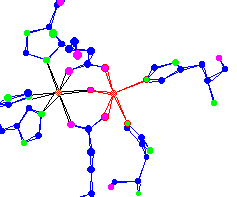 |
Trigonal Planar |
|
| Protoporphyrin IX of hemoglobin Hemoglobin is used for oxygen transport in humans. All around the plane of the protoporphyrin IX there are trigonal planar conformations. |
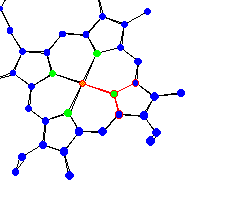 |
Trigonal Pyramidal |
|
| The 4 Sulfur of the (4Fe-4S) Ferredoxin center Used for electron transport Because of the nature of x-ray diffraction, hydrogens do not show up in protein data. So many molecules that might appear to be trigonal pyramidal are in fact tetrahedral. |
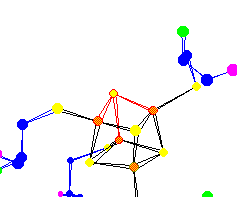 |