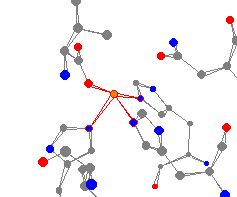
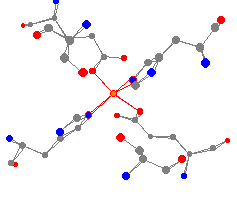
See-Saw
| This iron center comes from the protein 2,3-dihydroxybiphenyl dioxygenase. | 
| Dioxygenase is important in the breakdown of widely found environmental pollutants PCBs. It catalyzes the extradiol ring cleavage of 2,3-dihydroxybiphenyl. |
Trigonal bipyramidal iron is found in Fe(II) superoxide dismutase,
from E. coli.
Trigonal Pyramidal
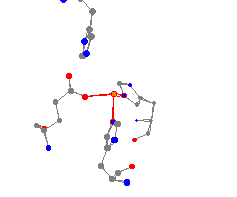
This iron center is found in the protein soy bean lipoxygenase L3.
This enzyme plays a key role in the metabolism of unsaturated fatty
acids. (It is likely that there are missing water molecules from the
reported structure, which would give the iron center a different geometry.)
Trigonal Bipyramidal
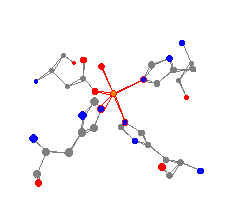
This enzyme catalyzes the dismutation of superoxides, for example HO2
and O2-.
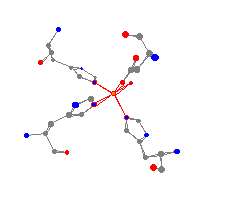
models it is possible to see the differences between a molecule
containing an
Fe(II) and an Fe(III). As can be seen when the models are constructed,
the Fe(III)
clearly has the surrounding atoms closer than the Fe(II).
This is due
to the more positive character of the Fe(III) drawing the surrounding
atoms nearer.
Tetrahedral
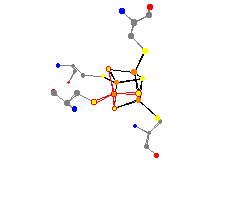
This interesting atom serves as a transition to the section of
iron-sulfur systems. Aldehyde ferredoxin oxidoreductase is an enzyme
that catalyzes the reversible oxidation of aldehydes to carboxylic
acids. It is a very interesting molecule because of its extreme
thermostability. This ferredoxin was discovered in the Archaebacteria
P. furiosus, and first grabbed the attention of scientists
because it contains the third-row transition metal tungsten. This
ferredoxin has two areas of iron interest. It has the single iron in
a tetrahedral geometry, and also has an Fe4S4
group. This second group contains four iron atoms in a structure with four
sulfur atoms.
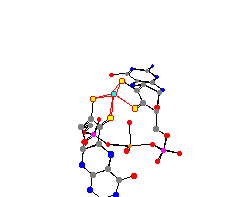
Octahedral
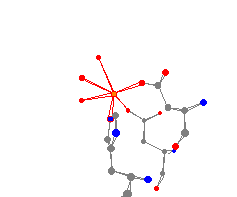
This trigonal pyramidal iron center comes from the molecule human
calcineurin heterodimer. The phosphatase not only contains iron, but also contains
several calcium atoms. Human calcineurin heterodimer is very important for several
cellular processes, including the activation of T-cells. The three water molecules
attached to the iron give this iron center a distorted octahedral geometry.
Iron-Sulfur Systems
Two Other Interesting Proteins